Apis Iq Fmea Software Free
FMEA Solutions provides some useful information about the different parts of the IQ Software including explanations on the CARM and CARM NG Server.
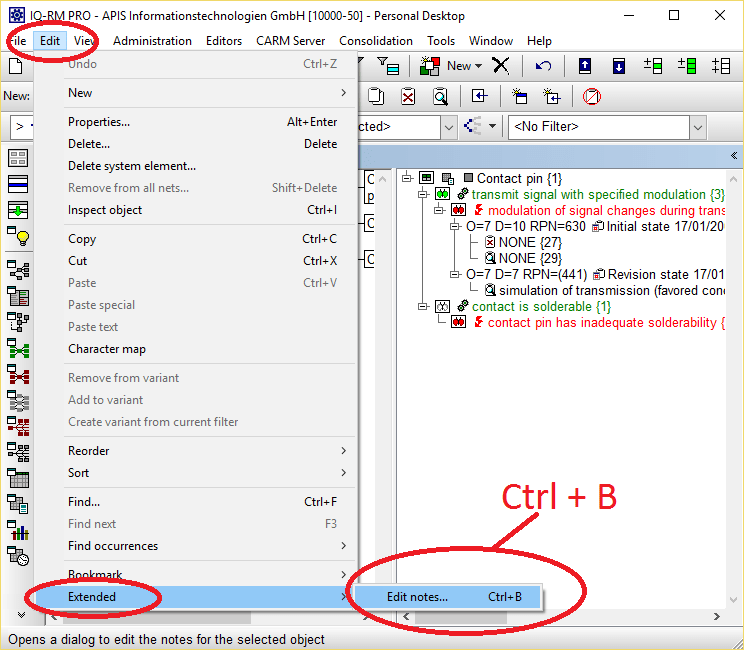
ReliaSoft's Xfmea software facilitates data management and reporting for failure mode and effect analysis ( FMEA and FMECA) and related analyses. The GMP Guideline Database contains more than 1,200 GMP Guidelines e.g.
The Xfmea Software Tool Supports All Types of FMEA and FMECA. Relia. Soft. com >. Reliability Software Tools >. Xfmea > Product Features. Relia. Soft’s Xfmea supports the major industry standards for all. FMEA analysis (including Design FMEA, Process FMEA, FMECA. The software provides predefined profiles for the major.
APIS IQ Software is one of the most advanced softwares for both FMEA, DRBFM and Functional Safety on the market. Logos Bible Software Serial Number Crack Adobe. Try the 60 day free trial today. In this era of competition quality has been given prime magnitude; failure to meet such quality allied goals produces massive shift of company in share of market. Minitab unterstützt Unternehmen dabei, durch intelligente Datenanalysen die Effizienz zu steigern und die Qualität zu verbessern.
Xfmea enables you to transfer relevant data between. Design FMEAs and Process FMEAs, and also provides worksheets for related analyses such as Design Verification Plans. DVP& Rs), Design Reviews Based on Failure Mode (DRBFMs), Process. Flow Diagrams, Control Plans and P- Diagrams. Multi- User Access and Intelligent Integration Xfmea analyses are stored in a centralized database that supports. Synthesis- enabled software tools. This enables. you to build and manage a valuable, keyword- searchable knowledge.
For enterprise- level. Microsoft SQL Server.
You also can use fully. Excel. Multiple Views of the Analysis Information Xfmea's flexible. System Hierarchy panel provides a uniquely powerful opportunity to. Then for each analysis, you can. FMEA data: the traditional worksheet, an intuitive hierarchical. RPN or. actions ranked by due date).
Support for Related Analyses. Xfmea provides integrated support for related analysis such as. Design Verification Plans (DVP& Rs), Design Reviews Based on Failure Mode (DRBFMs). Process Flow Diagrams (PFD Worksheets), Process Control Plans. PCPs) and P- Diagrams.
Feedback Loop for Corrective Actions To. FMEA, Xfmea provides a. This includes targeted reports, charts and the. Flexible. Reports, Queries, Charts and Diagrams Xfmea provides a complete set.
Quality by design approach: Regulatory need. In this era of competition quality has been given prime magnitude; failure to meet such quality allied goals produces massive shift of company in share of market. In this context pharmaceutical industry is utmost regulated industry as it is governed by authoritative regulatory bodies. USFDA launched a pilot programme in 2. CMC) of NDA information representing application of Qb. D. Now USFDA is accelerating Qb.
D drive by making warning to generic manufacturers from January 2. Qb. D has its perspectives to contribute the drug design, development, and manufacture of high- quality drug products. In the present review basic consideration of the Qb. D approach, its historical background, and regulatory needs are discussed. In detail explanation of elements of Qb. D i. e. Application of Qb. D to pharmaceutical and biopharmaceutical processes, development and unit operation associated with it are briefly mentioned.
Detail account of Qb. Cod 4 Memory Address Hack. D to analytical technique is explained thoroughly by referencing examples.